 |
SYDNEY, Dec. 1, 2021 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company developing next-generation products to address the growing needs in oncology, is pleased to announce that 15 of 30 participants have been recruited in the diagnostic 64Cu SAR-bisPSMA clinical trial (PROPELLER) in patients with untreated, confirmed prostate cancer, scheduled for radical prostatectomy.
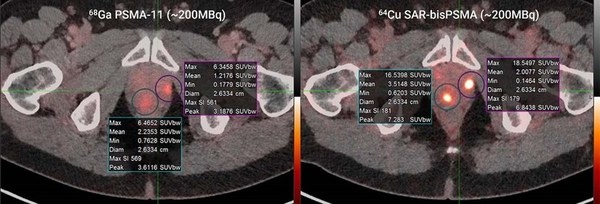
Ga-68 PSMA-11 (~200MBq, left) vs. Cu-64 SAR-bisPSMA (~200MBq, right) in the same patient; time between serial imaging was 8 days. Standardised Uptake Value (SUVmax)* of the lesions were 6.5 and 6.3 for Ga-68 PSMA-11 and 16.5 and 18.5 for Cu-64 SAR-bisPSMA
*SUV is a measurement of product uptake in tissue normalised to a distribution volume
Clarity's Executive Chairman, Dr Alan Taylor, commented, "We are excited to have quickly and successfully recruited half of the patients planned for the PROPELLER trial with all three sites actively recruiting and imaging prostate cancer patients across Australia. We have been able to not only generate strong preliminary clinical data since the trial commencement in July 2021, but also validate our on-demand distribution model where the 64Cu-SAR-bisPSMA has been shipped to the trial sites across Australia from a central manufacturing facility with minimal delays or interruptions. The pace and quality of work that we were able to achieve during this trial is reflective of the appetite for the new generation of radiopharmaceuticals that can cater to large indications in the oncology space and shift the radiopharmaceutical field towards the "big pharma" model with central manufacture of ready-to-use products. This shift has potential to significantly improve patient care by focusing on the needs of patients and enable their treating staff to access critical treatments that are safe and efficacious, on time and at any treatment centre with a positron emission tomography (PET) camera."
The PROPELLER trial is a Phase I Positron Emission Tomography (PET) imaging trial of participants with confirmed prostate cancer using 64Cu SAR-bisPSMA. It is a 30-patient multi-centre, blinded review, dose ranging, non-randomised study of 64Cu-SAR-bisPSMA administered to patients with confirmed prostate cancer prior to radical prostatectomy (NCT04839367)1. The main goals of the PROPELLER trial are to:
- Determine the safety and tolerability of 64Cu SAR-bisPSMA in participants with untreated, confirmed prostate cancer and planned for radical prostatectomy;
- Examine 64Cu SAR-bisPSMA at different dose levels;
- Determine the ability of 64Cu SAR-bisPSMA to detect primary prostate cancer; and
- Compare diagnostic properties of 64Cu SAR-bisPSMA against 68Ga PSMA-11, the standard of care for prostate cancer imaging in Australia.
Prof Louise Emmett (St Vincent's Hospital Sydney), Principal Investigator in the PROPELLER trial commented, "The preliminary data from the patients imaged in the PROPELLER trial to date looks very promising as it supports the evidence of higher uptake of 64Cu SAR-bisPSMA in the tumours that has been shown in the pre-clinical studies. Higher uptake in the tumours means that they are more visible on the PET scans and hence have a higher chance of being detected. These initial results are encouraging for further development of this product as a diagnostic, and the higher uptake and retention also make it an exciting therapeutic target with 67Cu. In addition to the anticipated clinical benefits, having access to centrally manufactured products has the potential to improve patient care by providing an alternative to currently used short-lived diagnostic radioisotopes, such as 68Ga and 18F, the production of which can pose challenges in delivering critical imaging scans to patients with cancer on time. I am very pleased to be working with Clarity on the Targeted Copper Theranostics (TCT) platform of products, having recently completed the C-BOBCAT trial of SAR-Bombesin, in hopes that these products will improve the current treatment paradigm for oncology patients."
Dr Alan Taylor further commented, "Clarity is well on track to explore and validate the benefits of the TCT platform, including the logistical, manufacturing and treatment benefits associated with the "perfect pairing" of copper-64 and copper-67. These benefits hold promise of providing a large patient population with early, accurate and precise detection of prostate cancer. As such, we look forward to recruiting the remaining 50% of patients and generating more data to validate the compelling results from our preclinical studies as well as the exciting preliminary results from the PROPELLER trial and our US-based 64/67Cu SAR-bisPSMA theranostic trial (SECuRE trial (NCT04868604)2) in pursuit of our ultimate goal of improving treatment outcomes for children and adults with cancer."
This announcement has been authorised for release by the Executive Chairman.
For more information, please contact:
Clarity Pharmaceuticals
Dr Alan Taylor Simon Hinsley
Executive Chairman Investor/Media Relations
ataylor@claritypharm.com simon@nwrcommunications.com.au
+61 401 809 653
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing targeted copper theranostics based on its SAR Technology Platform for the treatment of cancer in children and adults.
www.claritypharmaceuticals.com
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death worldwide3. The National Cancer Institute estimates in 2021 there will be 248,530 new cases of prostate cancer in the US and around 34,130 deaths from the disease4.
References
- ClinicalTrials.gov Identifier: NCT04839367 https://clinicaltrials.gov/ct2/show/NCT04839367
- ClinicalTrials.gov Identifier: NCT04868604 https://clinicaltrials.gov/ct2/show/NCT04868604
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660
- American Cancer Society, Cancer Statistics Center, https://cancerstatisticscenter.cancer.org/?_ga=2.79808020.284532473.1620009137-1916069442.1615761164#!/cancer-site/Prostate
Related Links :
https://www.claritypharmaceuticals.com
