HSA issues alert on 2 health products from Malaysia containing steroids
SINGAPORE - A woman was admitted to the intensive care unit (ICU) and a man developed Cushing's syndrome, after consuming two health products found to contain prohibited Western ingredients.
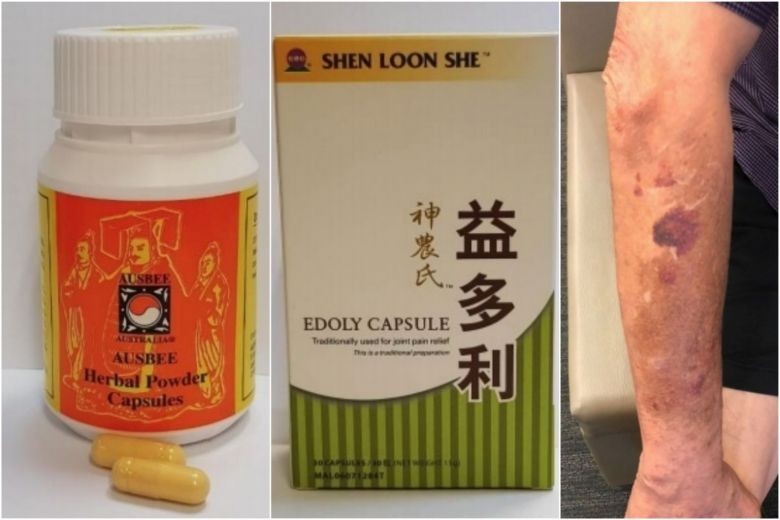
According to a statement from the Health Sciences Authority (HSA) on Monday (May 14), the two products -- Ausbee Australia Ausbee Herbal Powder Capsules and Shen Loon She Edoly Capsule, were obtained from Malaysia and taken for pain relief.
A woman in her 70s had been consuming Ausbee Australia Ausbee Herbal Powder Capsules for about two years to relieve her back pain and for general well-being.
The product was obtained from Malaysia through her friends. She underwent surgery due to a fracture and suffered complications arising from an adrenal crisis which caused a severe drop in her blood pressure. She was then admitted to the ICU.
Her condition was subsequently traced to the long-term consumption of dexamethasone, a potent steroid present in the capsules.
In addition to the steroid, "the product is also adulterated with antibiotics, a painkiller and an anti-allergy drug which can cause serious adverse reactions and drug interactions", said the HSA.
He developed Cushing's syndrome, a condition caused by long-term consumption of steroids.
He had symptoms characteristic of this condition - skin thinning, large purplish bruises on the skin, high blood pressure, and elevated blood glucose level. He is currently being treated for the condition.
Consumers who have taken the above products are advised to see a doctor as soon as possible, advised HSA.
Discontinuation of steroids without proper medical supervision can cause serious withdrawal symptoms such as fatigue, confusion, and low blood pressure, especially when the product has been taken for more than a few weeks, said HSA.
HSA also urged consumers to be wary of health products that promise quick results for chronic conditions and products from unfamiliar sources.
It added that sellers and suppliers must stop selling the two products immediately. Anyone found to be supplying illegal health products may be jailed for up to three years and/or fined up to $100,000.
a1admin@sph.com.sg